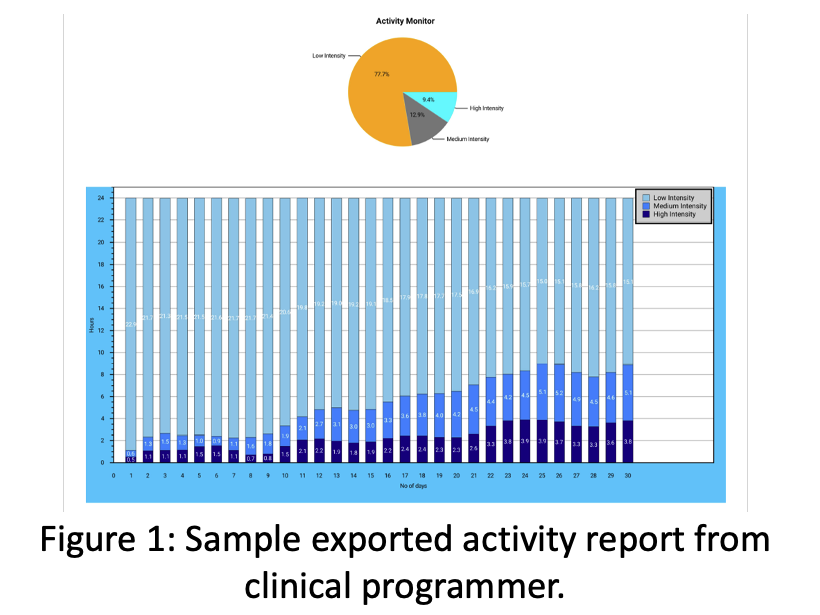
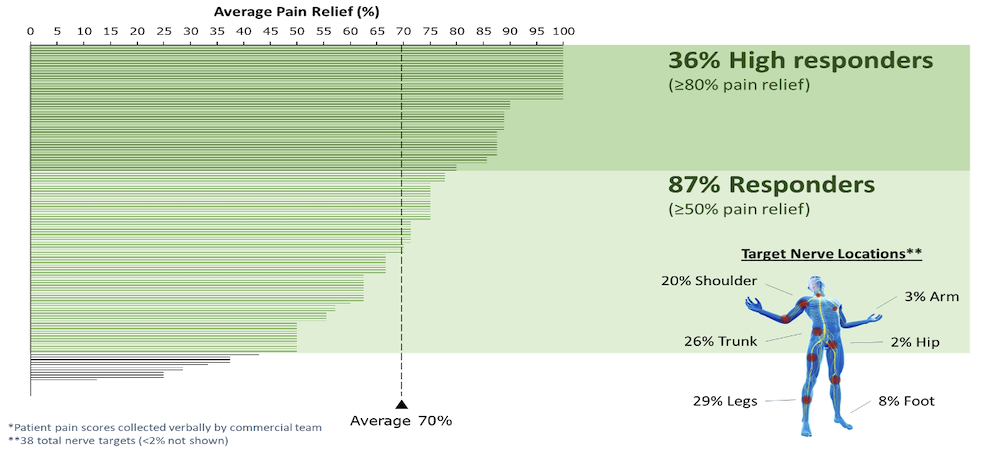
Nalu Medical, Inc. Closes $85 Million Series E Round
-
B Capital invests incremental $20M; previously raised $65M led by Novo Holdings with participation from existing investors
-
Equity investment to advance treatment for chronic neuropathic pain, accelerate commercial growth, and expand clinical and health-economic evidence for the innovative Nalu Neurostimulation System
CARLSBAD, Calif (BUSINESS WIRE) March 5, 2024
Nalu Medical, Inc. (Nalu), a private company focused on innovative, minimally invasive, and non-pharmacologic solutions for chronic neuropathic pain, announced today the closing of an incremental $20M in equity financing from new investor B Capital, bringing the total Series E round of equity investment to $85M. Series E was led by Novo Holdings (Novo) with $65M of the total closing in late-December 2023. Also participating in the round were all existing significant investors: Gilde Healthcare, MVM Partners, Endeavour Vision, Decheng Capital, Longitude Capital, Advent Life Sciences, Pura Vida, and Aperture Venture Partners.
“The Nalu Team is committed to addressing the unmet needs of patients suffering from chronic intractable neuropathic pain. We believe that our highly capable technology and uniquely small implant will elevate the standard of care in peripheral nerve stimulation (PNS) while being disruptive to the established spinal cord stimulation (SCS) market,” said Tom West, President and CEO of Nalu. “We are proud that our efforts serve the greater well-being of patients who suffer from chronic pain, and we are grateful for B Capital’s support in fulfilling and accelerating our mission.”
“The unique, clinically proven Nalu Neurostimulation System has the potential to address the unmet needs of millions who suffer from chronic neuropathic pain,” said Dr. Robert Mittendorff, Head of Healthcare and General Partner at B Capital. “We look forward to partnering with Nalu’s talented management team and strong syndicate of investors at a time of high revenue growth and potential for the company.”
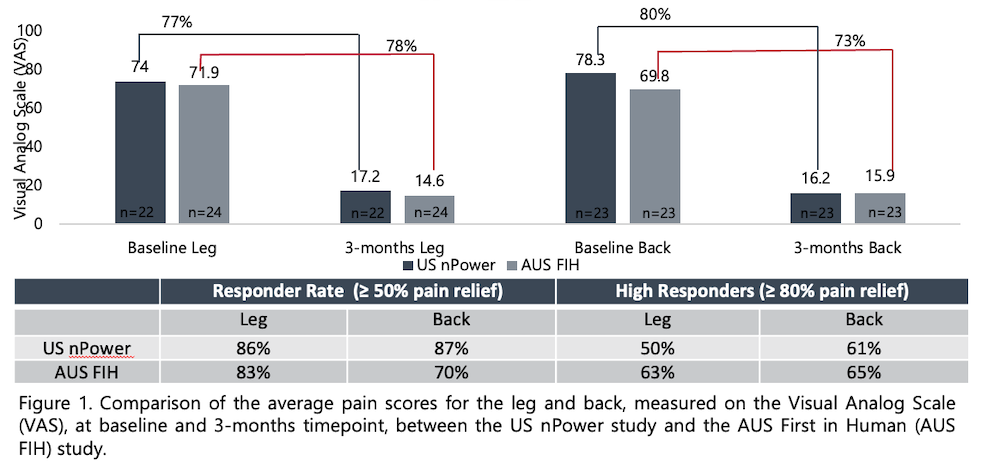
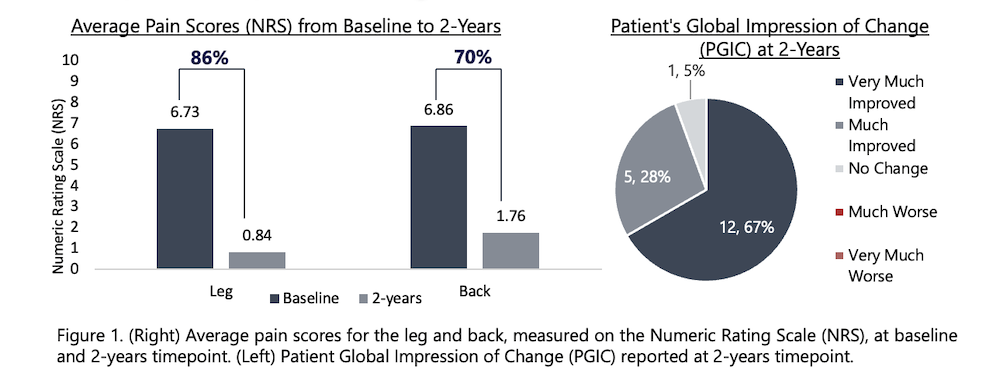
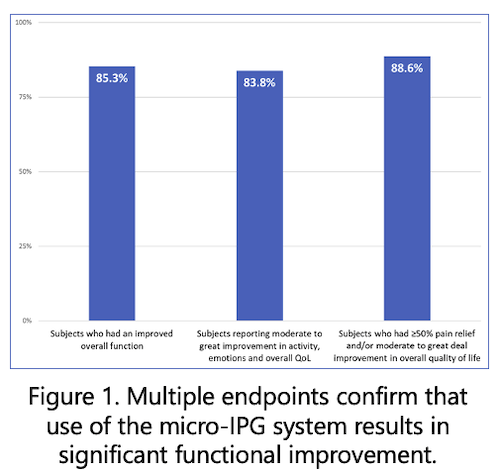
The proceeds from this financing will be used to accelerate commercial growth, expand clinical and health-economic evidence, continue product development, and scale operations. Nalu will continue to invest in building clinical data that provide physicians with clear evidence of its benefits while also serving as the basis for expanded access and payor coverage.
About Nalu Medical
Nalu is a Carlsbad, California-based medical technology company focused on developing and commercializing innovative and minimally invasive solutions for patients with chronic neuropathic pain. The Nalu Neurostimulation System delivers gentle electrical pulses to the nervous system to modulate pain signals before they get to the brain. The Nalu System was designed to address major unmet needs in the treatment of chronic neuropathic pain and provide a differentiated value proposition for patients and physicians.
About the Nalu Neurostimulation System
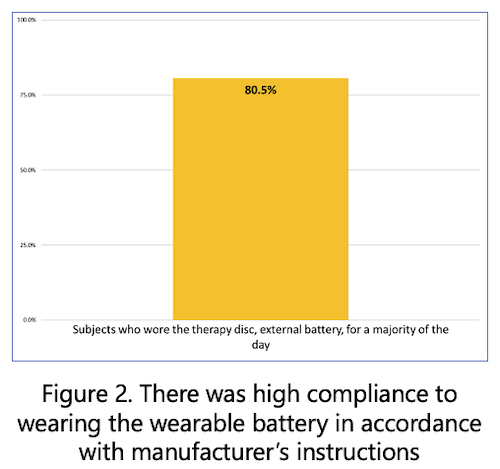
The Nalu System consists of a fully featured, battery-free, miniaturized implantable pulse generator (IPG) that is powered wirelessly by an externally worn Therapy Disc and controlled through a smartphone-based remote-control app. Despite its small size, the Nalu micro-IPG™ delivers treatment capabilities similar to larger IPGs as well as unique advantages associated with advanced waveforms, extensive programming options, exceptional upgradability, and an expected service life of 18 years. The Nalu System has been repeatedly recognized for its revolutionary technology, including being named as one of the world’s top 100 new products by R&D Magazine in 2021. It is FDA-cleared for Spinal Cord Stimulation (SCS) and Peripheral Nerve Stimulation (PNS) indications. To learn more, visit www.nalumed.com.
Indications for Use
Spinal Cord Stimulation — The Nalu SCS System is indicated as the sole mitigating agent or as an adjunct to other modes of therapy used in a multidisciplinary approach for chronic intractable pain of the trunk and/or limbs, including unilateral or bilateral pain. The trial devices are solely used for trial stimulation (≤ 30 days) to determine efficacy before recommendation for a permanent (long-term) device.
Peripheral Nerve Stimulation — The Nalu PNS System is indicated for pain management in adults who have severe chronic intractable pain of peripheral nerve origin as the sole mitigating agent or as an adjunct to other modes of therapy used in a multidisciplinary approach. The Nalu Neurostimulation System for PNS is not intended to treat pain in the craniofacial region. The trial devices are solely used for trial stimulation (≤ 30 days) to determine efficacy before recommendation for a permanent (long-term) device.
Nalu, the Nalu logo, and micro-IPG are trademarks of Nalu Medical, Inc.
About B Capital
B Capital is a multi-stage global investment firm that partners with extraordinary entrepreneurs to shape the future through technology. With $6+ billion in assets under management across multiple funds, the firm focuses on seed to late-stage venture growth investments, primarily in the enterprise, financial technology, and healthcare sectors. Founded in 2015, B Capital leverages an integrated team across nine locations in the US and Asia, as well as a strategic partnership with BCG, to provide the value-added support entrepreneurs need to scale fast and efficiently, expand into new markets, and build exceptional companies. For more information, visit www.b.capital.
Media Contact
Nalu Medical, Inc.
Jon Ruais
jon@nalumed.com