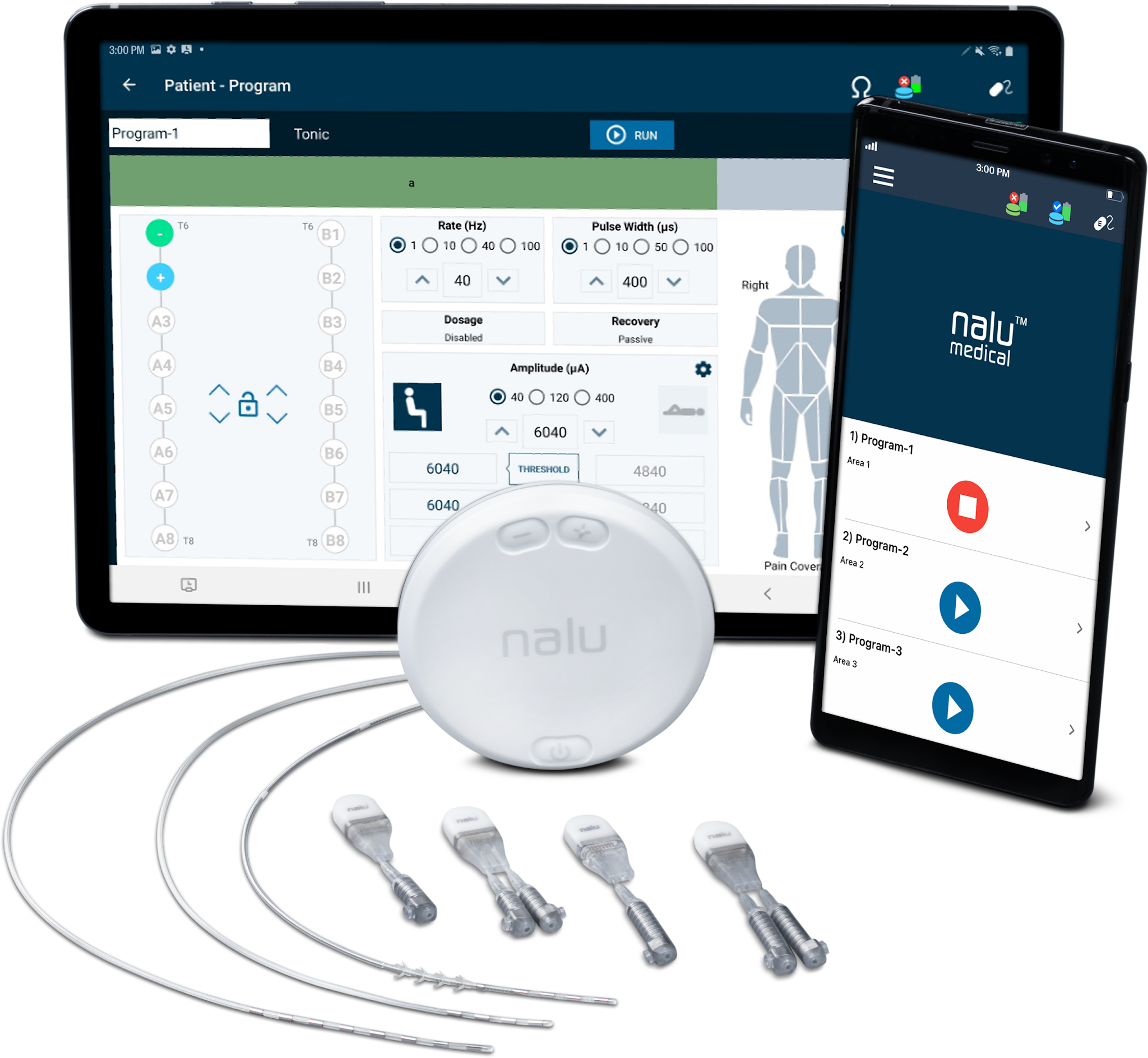
Nalu is setting a new standard for PNS outcomes
Results from COMFORT randomized control trial (RCT)( 1 ) have been published.
- Post-market, multicenter, randomized controlled trial
- Protocol designed with payor input
- Evaluated pain reduction and functional outcomes
- Inclusion criteria: post-surgical/post-traumatic peripheral neuralgia including pain due to peripheral nerve injury.
- Modified Intent to treat (mITT) population = 77
- 42 subjects randomized to PNS + conventional medical management
- Hatheway J Hersel A, Song J, et al Clinical study of a micro-implantable pulse generator for the treatment of peripheral neuropathic pain: 3-month and 6-month results from the COMFORT-randomized control trial Regional Anesthesia & Pain Medicine Published Online First: 31 May 2024. doi: 10.1136/rapm-2023-105264
Responder Rate at 6 Months of PNS use
(≥ 50% pain relief)
Numeric Rating Scale (NRS)
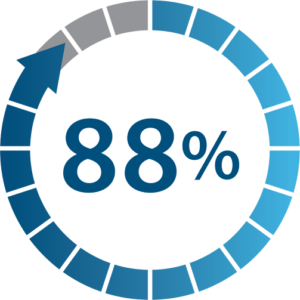
Nearly 9 out of 10 patients experienced positive results using Nalu PNS. An average of 70% pain reduction was reported for all patients.
Percentage of patients reporting improvement
Patient global impression of change (PGIC)
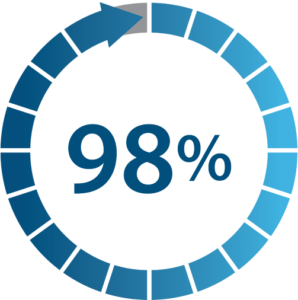
98% of patients reported overall improvement using Nalu PNS. In addition, patients reported clinically important improvements( 2 ) in disability, mood, quality of life, and pain interference.
2. Data on File, Nalu Medical
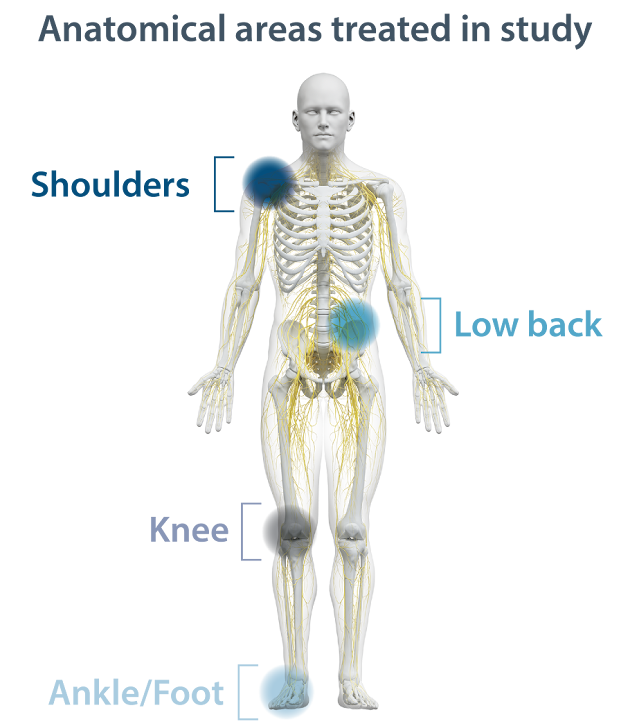
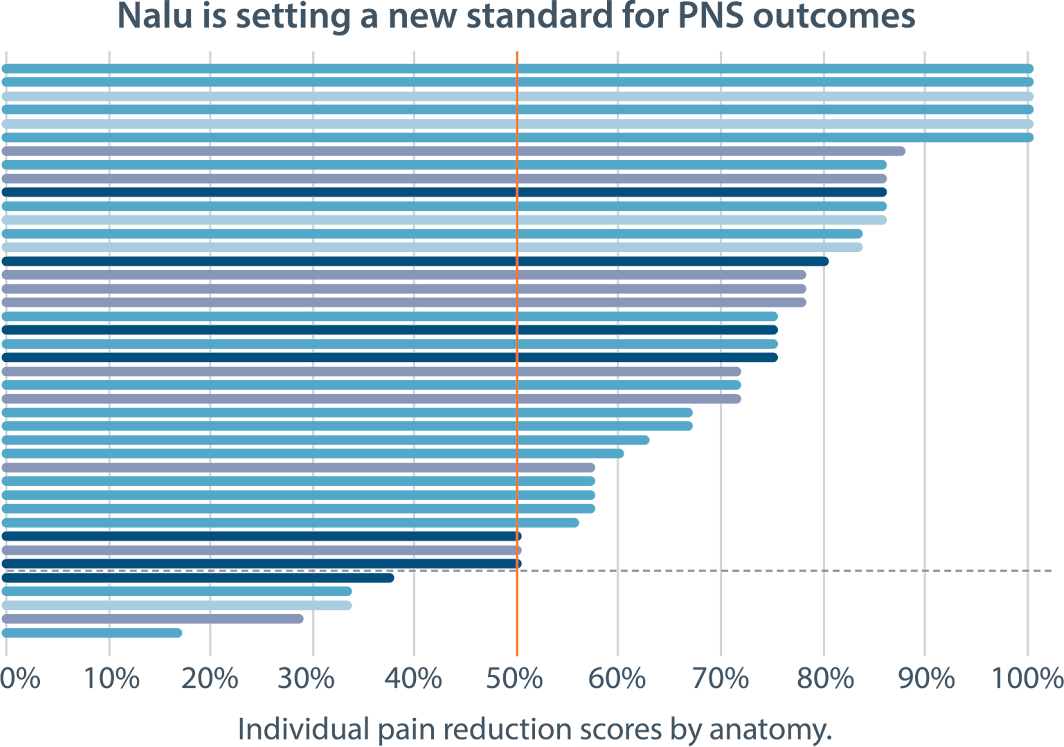
Nalu PNS shows durable pain relief
across a variety of pain areas
Tornado chart representing each study subject's
6-month pain outcome with PNS.
| SHOULDER | LOW BACK | KNEE | ANKLE/FOOT |
| SHOULDER | LOW BACK |
| KNEE | ANKLE/FOOT |
Nalu Outcomes only with Nalu Technology
Nearly all patients in the study utilized Nalu PNS capabilities typically seen only in SCS systems.
Study subjects employed at least one of the following:
- Pulse widths ≥500 μs.
- Frequencies ≥500 Hz.
- Multi-electrodes.
- Multi-area (including cross lead stimulation).
- Scheduling.
- Proprietary Nalu waveforms (current steering, PSP, pulse width >1000 μs).