Nalu Medical Scientific Presentations At ASPN Annual Conference 2022
Nalu Medical, Inc. gave the following five scientific presentations during the 2022 American Society of Pain and Neuroscience (ASPN) Annual Conference in Miami Beach, Florida. For more information, please reach out to the Nalu Medical team.
Comparison of Outcomes in Australian and US Subjects Implanted with A Micro-IPG SCS System
Authors: MJ. Desai, S. Arulkumar, J. Salmon, P. Verrills, G. Heit, S. Kottalgi.
Affiliations: International Spine, Pain and Performance Center, Washington, D.C, USA; SSM Health, Pain Management, Oklahoma City, OK, USA. Pain Care Perth, Pain Management, Perth, Australia. Metro Pain Group, Pain Management, Melbourne, Australia. Department of Neurosurgery, Kaiser Permanente-The Permanente Medical Group (TPMG), Redwood City, CA, USA. Nalu Medical, Inc., Carlsbad, CA, USA.
Introduction
A battery-free, spinal cord stimulation (SCS) system with a micro-implantable pulse generator (micro-IPG) (Nalu Medical, Inc., Carlsbad, CA) delivers various therapy options (traditional tonic, proprietary Pulsed Stimulation Patterns (PSP), current steering, burst waveforms, multi-area programs, scheduled therapy, and combinations and paired therapies).
Methods
Two contemporaneous, prospective, multi-center clinical studies were initiated in the US (US nPower) and Australia (AUS-FIH) to evaluate the micro-IPG SCS system in subjects with chronic pain of the leg(s) and/or low back. Both studies had similar eligibility criteria and included a Visual Analog Scale (VAS) to record pain as an endpoint. Per each protocol, following consent, data including VAS, was obtained at baseline and 3-month post-activation of the permanent implant. Both studies were conducted per applicable local guidelines with approval from Ethics Committees.
Results
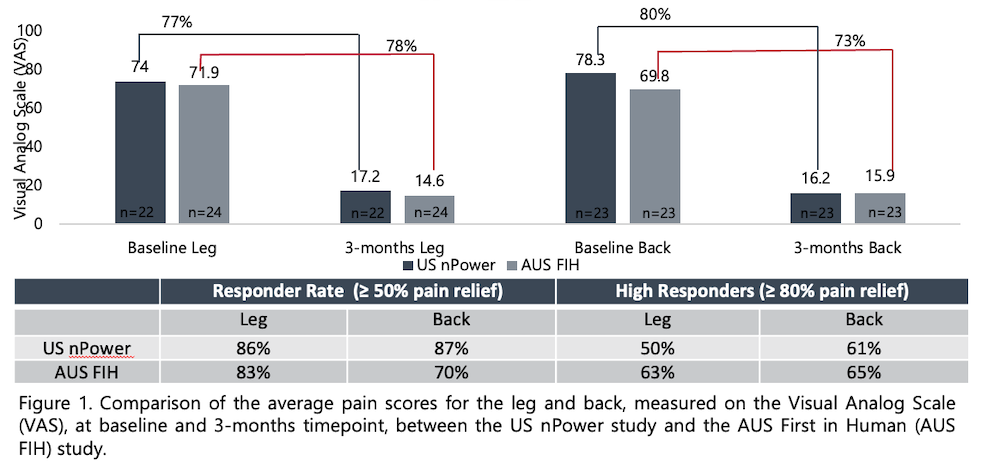
To-date, 3-month data is available on a total of 46 subjects, 24 in Australia and 22 in US. Concordant outcomes were noted in responder rates (percentage of subjects with ≥ 50% pain relief from baseline to 3-months) (AUS=83% [20/24], US=86% [19/22]) and improvement in VAS scores (AUS=78%, US=77%), in the leg(s). Similar agreement in improvement in VAS for lower back (AUS=73%, US=80%) was noted, while the US study showed a better responder rate (AUS=70% [16/23], US=87% [20/23]; 1 patient had no back pain at baseline in AUS). Both studies had no reports of pocket pain nor serious adverse device effects. A majority of patients in both studies used some form of PSP (PSP alone, scheduled, layered with tonic), even though they were each provided a choice of many therapy options. Both studies showed improvements in patient reported functional outcomes. The Beck’s Depression Inventory showed a 48% improvement in the Australia cohort, while the US subjects showed a 78% improvement, at 3-months. The Oswestry Disability Index showed a similar trend, with subjects in Australia reporting a 38% improvement compared to the 55% improvement seen in the US study. In addition, the reported average comfort scores were <1, on a 0-11 Likert scale with 0= Very Comfortable and 10=Very Uncomfortable, in both Australia and US subjects.
Conclusions
While this is not a formal meta-analysis, the similar outcomes of the two studies conducted in culturally and geographically diverse populations, may provide some indication of consistency of results. Data from two studies indicate that use of the micro-IPG system, with its multiple therapy options, may produce significant reductions in pain. Both studies are ongoing and additional investigation is warranted to confirm these findings.
Two-Year Results of an Externally Powered, Micro-implantable Spinal Cord Stimulator For Treatment Of Chronic Pain
Authors: Bates, MBBS; Verrills, MBBS; Salmon MBBS; Yu, MD; Mitchell, MBBS; Du Toit, MBChB; Green, BMBS; Taverner, MBBS; Mohabbati, MD; Peter Staats, MD; Gary Heit, PhD, MD; Levy MD, PhD; Kottalgi
Affiliations: Metro Pain Group, Pain Management, Melbourne, Australia. Pain Care Perth, Pain Management, Perth, Australia. Sydney Spine and Pain, Pain Management, Sydney, Australia. Pain Medicine of South Australia, Pain management, Adelaide, Australia. Frankston Pain Management, Pain Management, Frankston, Australia. Sydney Pain Management Centre, Pain Management, Sydney, Australia. Premier Pain Centers, Shrewsbury, NJ USA. Department of Neurosurgery, Kaiser Permanente-The Permanente Medical Group (TPMG), Redwood City, CA, USA. Institute for Neuromodulation, Neurosurgery, Boca Raton, FL USA. Nalu Medical, Carlsbad, CA, USA.
Introduction
A spinal cord stimulation (SCS) system with a battery-free micro-implantable pulse generator (micro-IPG; Nalu Medical, Inc. CA, USA) is available for the treatment of intractable chronic pain. The system utilizes an external power source that bi-directionally communicates with the micro-IPG (~1.5 cc volume).
Methods
A prospective, multi-center clinical study was initiated to confirm the safety and performance of this system, in the treatment of intractable pain of the trunk and/or limb(s). Specifically, subjects with leg(s) and/or back pain, meeting eligibility criteria were recruited and consented into the study. Subjects underwent a SCS trial utilizing a menu of therapy options, including tonic and the Pulsed Stimulation Pattern (PSP) therapy. Eligible subjects received the permanent implant and were followed at pre-determined timepoints. This abstract reports on the 2-year pain relief and functional outcomes (depression, activities of daily living, overall change in quality of life). The study was approved by an independent Ethics Committee and conducted in compliance with local regulations.
Results
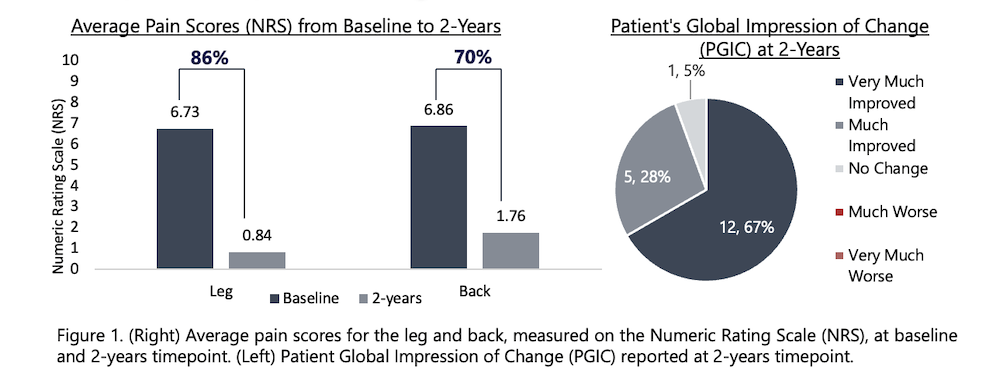
Twenty-one (21) subjects have completed 2-years of device use in this ongoing study. Due to COVID restrictions and other comorbidities, NRS pain diary and/or questionnaire data was not reported by 5 of the 21 subjects. In the remaining 16 subjects, leg(s) pain decreased from an average of 6.7 ± 1.3 at baseline to 0.8 ± 0.9 at 2-years; similarly, low back pain decreased from an average of 6.9 ± 1.2, at baseline, to 1.8 ± 1.8 at 2-years. The average percent pain reduction was 86% in the leg and 70% in the back, from baseline to this time point. The responder rate (≥50% pain relief from baseline) was 94% (15/16) in the leg and 73% (11/15) in the back. Of note, 81% (13/15) subjects with leg(s) pain were high-responders (≥ 80% pain-relief). The Beck’s Depression Inventory (BDI) and Oswestry Disability Index, both showed a 45% improvement from screening to 2-years. Ninety-four percent (94%; 17/18) of subjects indicated “very much improved” or “much improved” on the Patient Global Impression of Change.
Conclusions
These results continue to demonstrate the favorable long-term performance of this battery-free, externally powered micro-implantable SCS system. Further investigation is warranted to confirm these preliminary findings.
Real-World Survey of Chronic Pain Patients with a micro-IPG System and External Wearable Battery Source
Authors: J. Lefkovitz; J. Ruais, S Kottalgi, P. Martin
Affiliations: Nalu Medical Inc., Carlsbad, CA, USA.
Introduction
Nerve stimulation to treat pain was first mentioned in the literature in the 1960s. Technical advancements have allowed for the miniaturization of fully featured implantable pulse generators to less than 2cc in volume. A novel neuromodulation system with a micro-implantable pulse generator (micro-IPG) and external, wearable battery source (Nalu Medical, Inc., Carlsbad, CA) has FDA 510k clearances for spinal cord stimulation (SCS) and peripheral nerve stimulation (PNS). This micro-IPG system has options for multiple electrodes, bi-directional communication, and advanced programming options.
This is the first report of a large-scale real-world survey to collect patient reported outcome data on system use compliance, pain response, and functional improvement in micro-IPG patients.
Methods
One hundred eighty-five (185) subjects with either SCS or PNS micro-IPG implants were consented. Surveys were then administered via a secure and HIPAA compliant survey portal. Subjects answered standardized questions regarding the amount of pain relief achieved, changes in their level of activities, emotional status, overall quality of life, compliance in following wear instructions and general satisfaction with the therapy device.
Results
185 patients were consented and entered into the database.
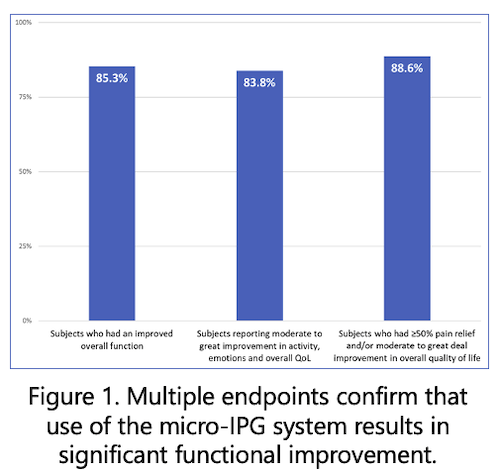
- 85.3% (157/184*) reported an improvement to their overall function. (*one subject did not answer this question)
- 83.8% (155/185) reported moderate to great improvement in change in activity limitations, emotions, and overall quality of life.
- 88.6% (164/185) of respondents reported ≥50% pain relief and/or moderate to great deal improvement in overall quality of life.
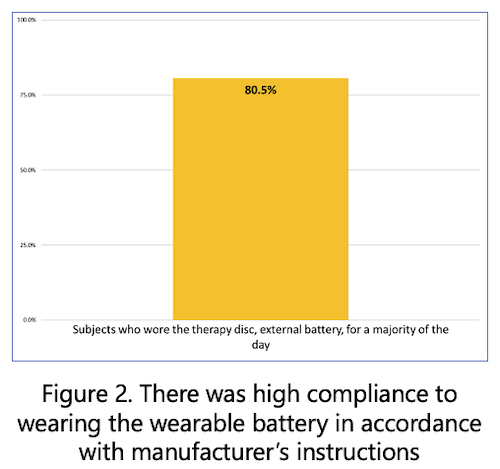
- 80.5% (149/185) of respondents reported that they wear the Therapy Disc, external battery, for a majority of the day.
Conclusions
The real-world patient reported outcome data suggests that use of the micro-IPG can significantly reduce pain, improve activity levels, and improve quality of life scores in a majority of patients. The data indicates that wearing the external battery does not interfere with the reported improvements in pain reduction and function. These promising results warrant further investigation.
Novel implementation of a 3-axis microelectromechanical system (MEMS) accelerometer within a wearable device for objective functional improvement measurement
Authors: Valimahomed, MD, FAAPMR; L. Mishra; K. King; M. Graves; A. Ding; C. Linden
Affiliations: Gramercy Pain Center, Red Bank, NJ, USA; Nalu Medical Inc., Carlsbad, CA, USA.
Introduction
Functional improvement data presents an additional tool to understand and communicate progress for pain patients. A neuromodulation system with an external wearable component (Nalu Medical, Inc. CA, USA) incorporates a 3-axis MEMS accelerometer which may be activated to monitor and record activity during use.
Activity is categorized into three discreet groups (sedentary, walking, running) via a custom algorithm and then presented within an activity report representing objective functional improvement information.
Methods
A validation effort was performed to qualify the hardware and refine the algorithm to accurately capture user activity. The accelerometer was configured such that it collects data at 50 Hz, where a ‘sample’ is an X, Y, and Z reading. The X, Y, and Z values are 10-bit resolution numbers which each map to a range of -4 g to +4 g. These inputs are read in another pass of the data to find the number and type of impacts seen in the set. This impact information is then used to update the current activity level of the patient.
Initially, six unique data sets were used to validate the model: Sample 1 – Stand, Medium Walk, Medium Jog, Medium Walk; Sample 2 – Fast Walk, Slow Jog, Stand, Sprint, Slow Walk; Sample 3 – Fast Walk, Slow Jog, Stand, Sprint, Slow Walk; Sample 4 – Medium Uphill Walk, Medium Downhill Run, Stand, Medium Uphill Run, Medium Downhill Walk; Sample 5 – Medium Treadmill Walk, Medium Treadmill Run, Stand, Medium Indoor Walk, Medium Indoor Stairs Walk; Sample 6 – Medium Treadmill Walk, Medium Treadmill Run, Stand, Medium Walk, Medium Run.
Subsequently, six adult participants wore a production version of the device for a multi-day comparison of reported activity versus device captured and categorized activity.
Results
The model correctly categorized all extended duration activities across all data sets. The largest challenges involved accurately assigning the data logged during the delay between an activity being started and its classification as that activity and transitions between activity types. This is due to the activity level requiring a certain number of consecutive periodic impacts before updating. However, occasional missed impacts are handled gracefully and do not commonly trigger false transitions to “sedentary”. The participant evaluation yielded acceptable alignment between reported and captured activities. Any perceived inaccuracies were not widespread or focused in scope, and were not enough to make the participants feel that their activity was being unreasonably represented.
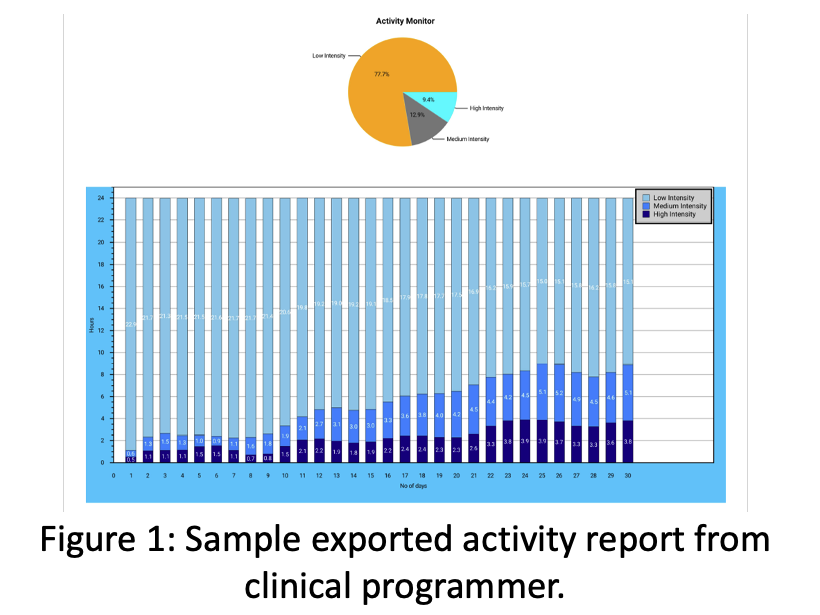
Conclusions
Validation efforts demonstrated system capability and accuracy in activity characterization across time and type. Activity measurement provides an additional metric for the evaluation of functional improvement for pain patients not susceptible to the subjective nature of commonly used self-reported data and may represent a useful assessment of progress.
First Results of a Real-World Survey of Patients Receiving Peripheral Nerve Stimulation to Treat Neuropathic Pain
Authors: J. Lefkovitz; J. Ruais, S Kottalgi, P. Martin
Affiliations: Nalu Medical Inc., Carlsbad, CA, USA.
Introduction
Peripheral nerve stimulation (PNS) is a well-established modality to treat severe intractable chronic pain of peripheral nerve origin. A novel neuromodulation system with a micro-implantable pulse generator (micro-IPG) and external, wearable battery source (Nalu Medical, Inc., Carlsbad, CA) has US market clearance for PNS and spinal cord stimulation (SCS) applications. This micro-IPG system has significant therapy options, with multiple electrodes, bi-directional communication, and advanced programming options. This is the first report of a large-scale survey of micro-IPG patients for PNS treatment of chronic pain.
Methods
One hundred ninety-eight (198) subjects with PNS implants provided consent to provide their clinical data to be housed in a company sponsored, secure, and controlled database; patients also agreed to be contacted by company representatives for telephonic follow up. Patient information was entered into the database through a secure web-based portal. Employees of the company contacted the patients for follow ups, and to collect data and resolve errors/omissions. Patients were asked a series of questions related to their pain profiles, quality of life, and overall satisfaction with therapy at various time points post implant of their micro-IPG system. The data collection is ongoing and will continue to house all commercial patients.
Results
- PNS therapy was used to treat thirty-eight (38) different nerve targets/nerve combinations. The top targets were legs (29%), trunk (26%), shoulder ( 20%), foot (8%), arm (3%), and hip (2%).
- 87% of all PNS patients had a successful treatment (defined as ≥50% pain relief), with an average pain relief across all patients of 70%.
- 36% of all PNS patients were high responders (defined as ≥80% pain relief).
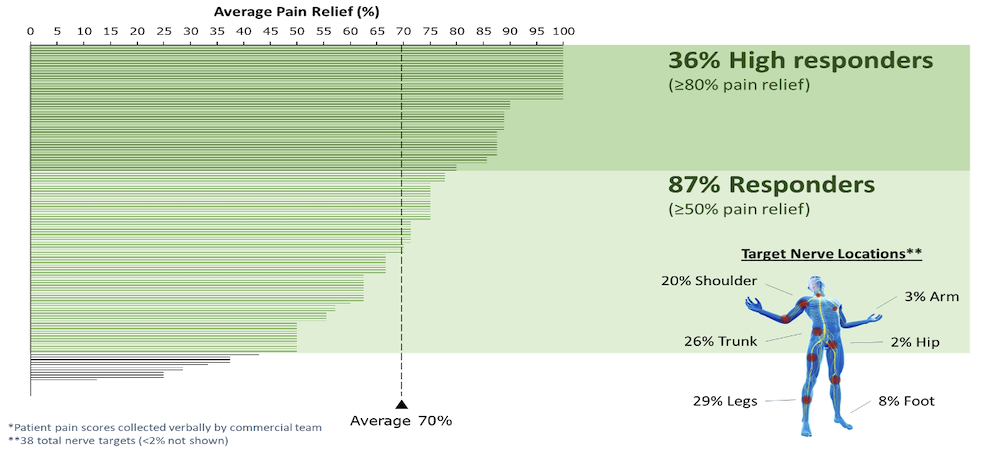
Conclusions
This data is capturing real-world patient reported outcomes following permanent implant of a micro-IPG PNS system. Limitations of this survey include a small sample size and the use of non-validated instruments to capture patient reported outcomes; this will be addressed through an updated, recently implemented survey. This data indicates that PNS therapy delivered by a micro-IPG system can produce pain relief and improvement in general quality of life in most patients at a level usually associated with the SCS literature. A registry will be implemented to further the study of these positive results in a larger population.