Nalu Medical, Inc., Announces Publication of Two Spinal Cord Stimulation Clinical Studies
Both studies report on clinical outcomes related to the use of the Nalu proprietary, multidimensional Pulsed Stimulation Pattern (PSP) waveform
CARLSBAD, Calif. (PRWEB) January 5, 2023
Nalu Medical, Inc. (“Nalu”), a Carlsbad, California-based company that has successfully miniaturized neurostimulation implants for the management of chronic intractable pain for both Spinal Cord Stimulation (SCS) and Peripheral Nerve Stimulation (PNS) indications, announces the publication of two key studies in Neuromodulation: Technology at the Neural Interface. One publication discusses the potential mechanisms of action (MOAs) leveraged by a novel, multidimensional waveform Pulsed Stimulation Pattern (PSP) and the results of a pilot clinical study. The other publication reports on interim results from a first-in-human SCS clinical study evaluating the safety and performance of the Nalu Neurostimulation System, composed of a micro-implantable pulse generator (micro-IPG™) and a wearable Therapy Disc (power source and stimulation control).
Following are some highlights of the PSP pilot study report on the theory behind and design of the multidimensional PSP waveform along with pilot study results from 31 patients with chronic intractable pain:
- The PSP waveform is composed of three independently configurable temporal dimensions designed to target different MOAs.
- PSP yielded a greater reduction in both back and leg pain than traditional SCS (Back: -60% vs. -46%; Legs: -63% vs. -43%
- PSP yielded higher responder rates for both back and leg pain compared with traditional SCS (61% vs. 48% and 78% vs. 50%, respectively).
“Up until now, neurostimulation waveforms for SCS have been a ‘one-note proposition’ for pain relief. One waveform leverages one unique MOA, much like playing a single musical note,” said Dr. Mehul J. Desai, President and Medical Director of the International Spine Pain & Performance Center and lead author of the PSP pilot study publication. “Pulsed Stimulation Pattern is more like playing a musical chord. Multiple MOAs are leveraged simultaneously for multimechanistic therapeutic relief. This represents an exciting new era for SCS, where we may provide patients with ‘broad-spectrum’ relief that may address their evolving pain patterns over the long term.”
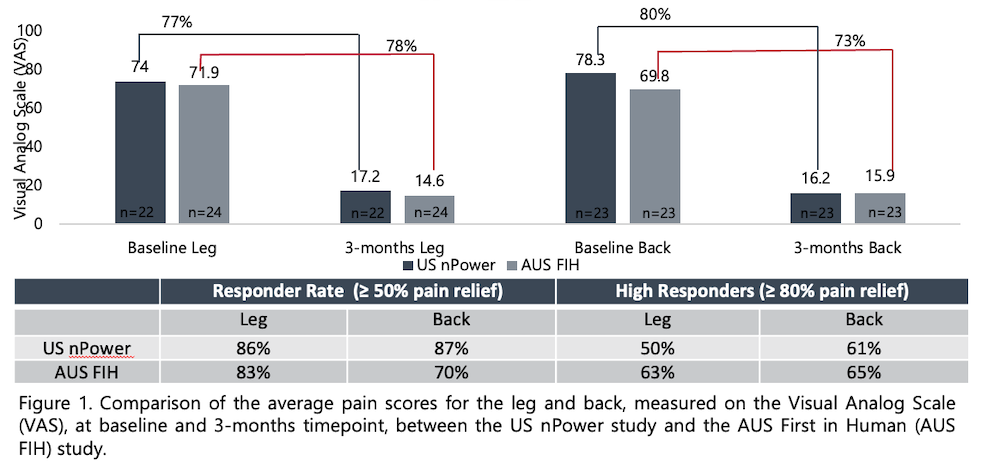
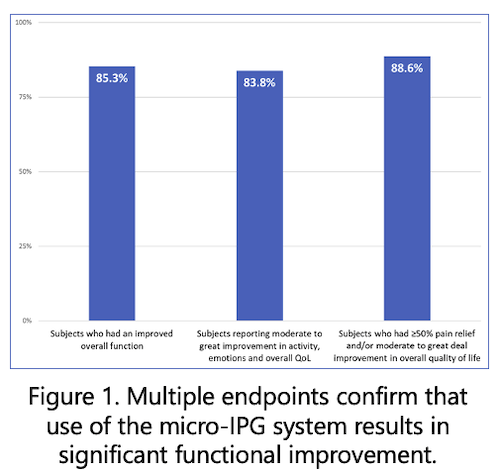
Following are some highlights of the first-in-human Nalu SCS clinical study report on the 90-day (three-month) outcomes of 22 subjects who chose the PSP waveform using the Nalu Neurostimulation System for treatment of chronic intractable leg and lower back pain:
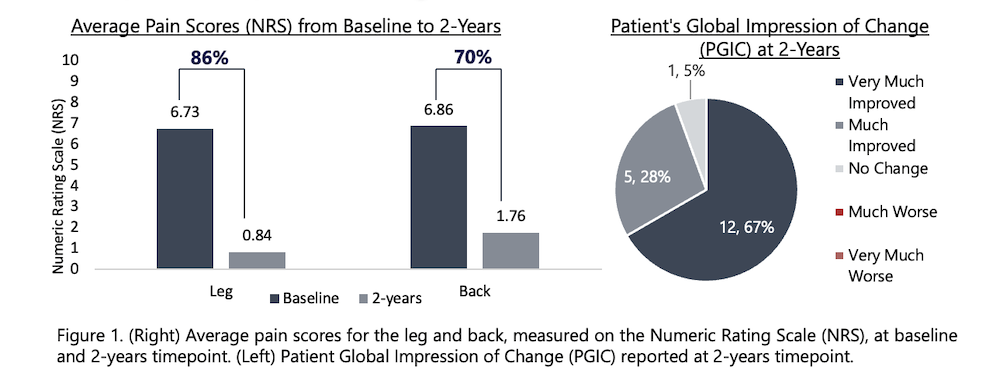
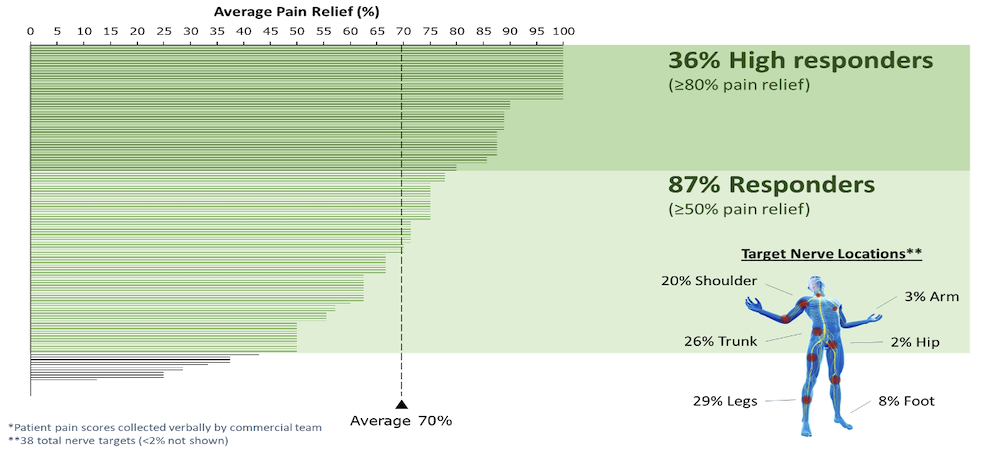
- At the 90-days follow-up visit, the average pain reduction was 79% in the leg and 76% in the lower back compared with baseline.
- Responder rates (≥50% pain relief) at 90 days were 86% in leg pain and 81% in lower back pain.
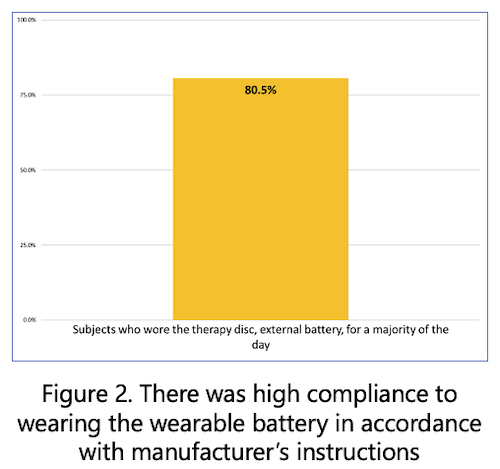
- Subjects rated the external wearable power source “very comfortable” throughout the study period.
“Fully implanted SCS systems are intuitively appealing, but they entail ongoing burdens to manage and risks to continually replace,” said Dr. John Salmon, pain medicine specialist at PainCare Perth and lead author of the first-in-human Nalu SCS clinical study publication. “The Nalu System defines a new standard for minimally invasive, lower-risk, and markedly more cost-effective neuromodulation treatment for a patient’s lifetime of managing chronic pain. Our study, and continued experience over more than three years to date, have demonstrated that patients respond favorably to a system with a tiny implant and a battery that may only need to be worn at intervals.”
About Nalu Medical
Nalu is a Carlsbad, California-based medical technology company focused on developing and commercializing innovative and minimally invasive solutions for patients with chronic neuropathic pain. The Nalu Neurostimulation System delivers gentle electrical pulses to the nervous system to modulate pain signals before they get to the brain. The Nalu System was designed to address major unmet needs in the treatment of chronic neuropathic pain and provide a differentiated value proposition for patients and physicians.
About the Nalu Neurostimulation System
The Nalu System consists of a fully featured, battery-free, miniaturized implantable pulse generator (IPG) that is powered wirelessly by an externally worn Therapy Disc and controlled through a smartphone-based remote control app. Despite its small size, the Nalu micro-IPG™ delivers treatment capabilities similar to larger IPGs as well as unique advantages associated with advanced waveforms, extensive programming options, exceptional upgradability, and an expected service life of 18 years. The Nalu System has been repeatedly recognized for its revolutionary technology, including being named as one of the world’s top 100 new products by R&D Magazine in 2021. It is FDA-cleared for Spinal Cord Stimulation (SCS) and Peripheral Nerve Stimulation (PNS) indications. To learn more, visit www.nalumed.com.
Indications for Use
Spinal Cord Stimulation — The Nalu SCS System is indicated as the sole mitigating agent or as an adjunct to other modes of therapy used in a multidisciplinary approach for chronic intractable pain of the trunk and/or limbs, including unilateral or bilateral pain. The trial devices are solely used for trial stimulation (≤ 30 days) to determine efficacy before recommendation for a permanent (long-term) device.
Peripheral Nerve Stimulation — The Nalu PNS System is indicated for pain management in adults who have severe chronic intractable pain of peripheral nerve origin as the sole mitigating agent or as an adjunct to other modes of therapy used in a multidisciplinary approach. The Nalu Neurostimulation System for PNS is not intended to treat pain in the craniofacial region. The trial devices are solely used for trial stimulation (≤ 30 days) to determine efficacy before recommendation for a permanent (long-term) device.
Nalu, micro-IPG, and the Nalu logo are trademarks of Nalu Medical, Inc.
Disclaimers
The specific parameters used in the commercial use of the Nalu Neurostimulation System have evolved over time and are now different from the early set of programming parameters used in these clinical studies.
Any medical opinions expressed are those of the physician/medical providers. Individual experiences and outcomes may vary.
Media Contact
Nalu Medical, Inc.
Jon Ruais
jon@nalumed.com
925-667-6329